Idea
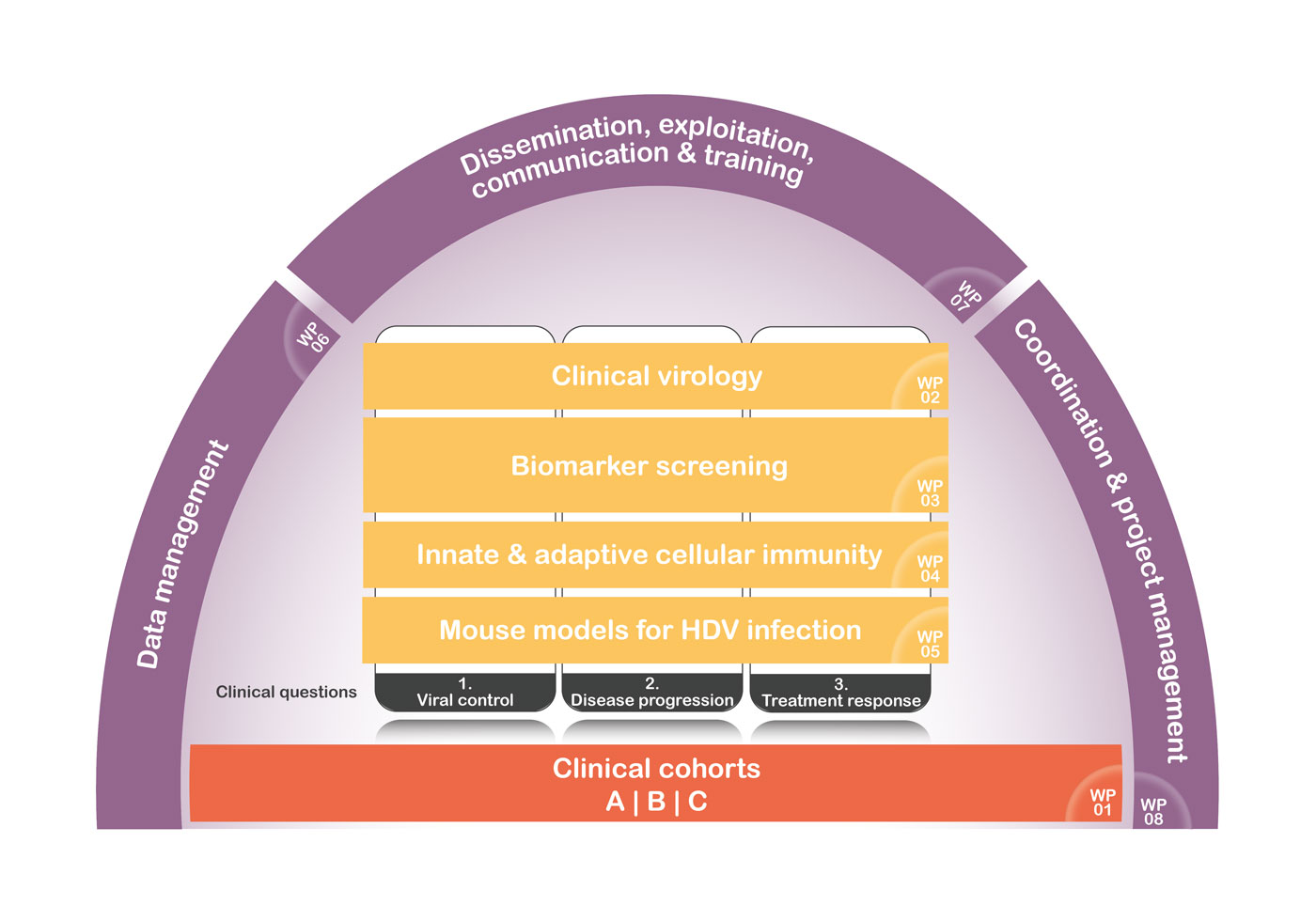
In undertaking a highly challenging project as the present one, we planned a work schedule over a 48- month project period. Interdependencies between the right research and development WPs will be ensured. To achieve the specific WP objective(s), the work plan activities are organized into eight Work Packages (WPs), each consisting of a number of associated tasks that collectively ensures the achievements of the project objectives. Progress against program, budget, and the obtained results will be monitored on a regular basis by the Project Steering Committee (PSC). Should it occur that any WP is late with respect to the programmed timing by more than one month, the Project Coordinator will bring it to the attention of the PSC, and a plan will be agreed and implemented to recover the delay.
In working towards personalized treatment of chronic hepatitis delta, the foundation of this project are large world-unique clinical cohorts (WP1) that will be utilized in upcoming WPs (2, 3, 4, and 5) in answering three key clinical questions on individual determinants of i) fibrosis progression, ii) HDV replication control, and iii) response to treatment. The project coordinator will produce the major periodic reports and minutes of progress meetings will give an update of the project progress at 6 monthly intervals during the program.
Work Package 1
Clinical cohorts
WP1 is the first work package and will lay the foundation for WPs 2 to 5 in assessing the specific scientific questions on i) control of HDV replication, ii) individual determinants of fibrosis progression, and iii) treatment response. Three subprojects will be established with the first collecting in a cross-sectional approach 750 patients reflecting the entire spectrum HDV infection in Europe, the second consisting of a retrospective-prospective cohort of patients with liver biopsies taken 10-20 years ago to allow the investigation of intrahepatic immune correlates with disease progression and the third will be a prospective trial of patients being treated with the novel entry inhibitors bulevirtide.
Work Package 2
Clinical virology: testing for novel virological markers; HDV & HBV sequencing
This work package will be essential to perform a comprehensive virological characterization of these patients who are infected with two viruses HBV and HDV. As local diagnostics in particular for HDV but also for novel HBV markers have not been fully standardized, central testing with optimized methodology will be required. In addition, WP2 will perform an in-depth viral sequencing of both HBV and HDV which will be crucial to determine immune pressure on the virus and to dissect host-viral interactions.
Work Package 3
Biomarker screening
WP3 starts with retrospective samples (cohort B) and will then continue with the other cohorts during the entire funding period. This work package will be the core project to identify novel biomarkers for the three key clinical challenges to be addressed in D-SOLVE. KI and HZI/CiiM will perform the respective screening in collaboration with MHH and INSERM.
Work Package 4
Innate and adaptive immunity
This work package led by KI and MHH will explore mechanisms of biomarkers identified in WP3 but also focus on hypothesis-driven questions, in particular investigating intrahepatic immune innate and adaptive responses. Intrahepatic immune responses have rarely been studied in hepatitis D and thus completely novel data can be expected which can probably only generated within this D-SOLVE consortium combining translational clinical and basic expertise and providing the logistics to have access to the required patient material.
Work Package 5
Mouse models of HDV infection and HDV-induced transcriptomic and epigenetic modifications
Given previous observations for HCV, it is likely that HDV control cure by either interferon-based therapies or in combination with novel antivirals like Bulevirtide will only partially eliminate HDV-induced modifications of the hepatocyte cell circuits or the innate and adaptive immune responses. These persistent perturbations of the liver cell circuits may contribute liver disease progression and HCC risk post cure. Supporting this concept, HDV induces DNA hypermethylation and histone modification in cell lines suggesting the existence of an epigenetic viral footprint relevant for viral pathogenesis and HCC risk. To address this question we will investigate HDV-induced transcriptomic and epigenetic modifications in liver tissues and PBMCs in a mouse model for HDV-HBV co-infection before and after antiviral therapies and then validate the identified modifications with samples from cohorts of WP1 and study their phenotype before and after antiviral treatment.
Work Packages 6-8
WPs 6 (Data management), 7 (Dissemination, exploitation, IP, communication & training) and 8 (Coordination & project management) will be carried out along the whole duration of the project.
Publications / Results
2026
Zulkhuu D, Kamal H, Enkhtaivan S, Narantsogt O, Jargalsaikhan G, et al. Prevalence of chronic viral hepatitis B, D among Mongol migrants in Sweden compared to a sex‑ and age‑matched native Mongol cohort. BMC Infect Dis. 2026 Jan 16;26(1):178. doi:10.1186/s12879‑025‑12506‑w Link to full text
—
Yurdaydin C, Kahlhöfer J, Caruntu F.A., Yalcin K, Gürel S, et al. Ten-Year Follow-Up After 96 Weeks Treatment With Peginterferon Plus Tenofovir in Hepatitis D (HIDIT‑II): Improved Clinical Outcome After Combination Therapy. United European Gastroenterol J. 2026 Feb;14(1):e70153. doi:10.1002/ueg2.70153 Link to full text
2025
Lampertico P, Anolli M.P., Steppich K, Wedemeyer H. Bulevirtide Monotherapy or in Combination for Chronic Hepatitis Delta: 2025 Update. J Viral Hepat. 2025 Dec;32(12):e70056. doi:10.1111/jvh.70056 Link to full text
—
Tonnerre P, Hoshida Y, Baumert T. Unraveling the genetic susceptibility to HBV‑related liver cancer: The role of virus‑host immune interactions. Hepatology. 2025 Apr 15:10.1097/HEP.0000000000001355. doi:10.1097/HEP.0000000000001355 Link to full text
—
Desert R, Gianonne F, Saviano A, Hoshida Y, Heikenwälder M, et al. Improving immunotherapy for the treatment of hepatocellular carcinoma: learning from patients and preclinical models. Gut Liver. 2025 Apr 3;2(1):s44355‑025‑00018‑y. doi:10.1038/s44355‑025‑00018‑y Link to full text
—
Wen R, Liu Y, Backes M, Zhang Y. SoK: Data Reconstruction Attacks Against Machine Learning Models: Definition, Metrics, and Benchmark. 34th USENIX Security Symposium, USENIX Association; pp. 5601–5620. doi:10.5281/zenodo.15603060 Link to full text
—
Wen R, Backes M, Zhang Y. Understanding Data Importance in Machine Learning Attacks: Does Valuable Data Pose Greater Harm? Network and Distributed System Security (NDSS) Symposium 2025. doi:10.14722/ndss.2025.230331 Link to full text
—
Wranke A, Wedemeyer H. Hepatitis D virus—still the devil! Hepatology. 2025 Jun 1;81(6):1643–1646. DOI: 10.1097/HEP.0000000000001144 Link to full text
—
Lampertico P, Anolli MP, Roulot D, Wedemeyer H. Antiviral therapy for chronic hepatitis delta: new insights from clinical trials and real-life studies. Gut. 2025 Apr 7;74(5):853–862. DOI: 10.1136/gutjnl-2024-332597 Link to full text
—
Degasperi E, Scholtes C, Testoni B, Uceda Renteria S, Anolli MP, Charre C, et al. Differential HBV RNA and HBcrAg patterns in untreated patients with chronic hepatitis delta. J Hepatol. 2025 Jun;82(6):1004–1011. doi:10.1016/j.jhep.2024.11.051 Link to full text
—
Hardtke S, Yurdaydin C, Caruntu FA, Curescu MG, Yalcin K, Akarca US, et al. Frequency, severity and impact of pegylated interferon alpha-associated flares in hepatitis D infection. J Viral Hepat. 2025 Apr;32(4):e70022. doi:10.1111/jvh.70022 Link to full text
—
Wedemeyer H, Leus M, Battersby TR, Glenn J, Gordien E, Kamili S, et al. HDV RNA assays: Performance characteristics, clinical utility, and challenges. Hepatology. 2025 Feb 1;81(2):637–650. doi:10.1097/HEP.0000000000000584 Link to full text
—
Chowdhury S, Jacobsen C, Depledge D, Wedemeyer H, Sandmann L, Kefalakes H. Sequence analysis of the hepatitis D virus across genotypes reveals highly conserved regions amidst evidence of recombination. Virus Evol. 2025 Feb 27;11(1):veaf012. doi: 10.1093/ve/veaf012 Link to full text
—
Degasperi E, Anolli MP, Jachs M, Reiberger T, De Ledinghen V, Metivier S, et al. Real-world effectiveness and safety of bulevirtide monotherapy for up to 96 weeks in patients with HDV-related cirrhosis. J Hepatol. 2025 Jan 8;S0168-8278(25)00001-7. doi:10.1016/j.jhep.2024.12.044 Link to full text
—
Chen P, Deterding K, Engelskircher S, Port K, Sandmann L, Chakkadath A, et al. TIGIT expression on natural killer cell subsets is an early indicator of alleviating liver inflammation following bulevirtide treatment in chronic hepatitis D. Hepatology. 2025 Jan 23. doi:10.1097/HEP.0000000000001238 Link to full text
—
Sandmann L, Ohlendorf V, Ehrenbauer A, Bremer B, Kraft A, Cornberg M, et al. Value and kinetics of virological markers in the natural course of chronic hepatitis D virus infection. Liver Int. 2025 Feb;45(2):e70003. doi: 10.1111/liv.70003 Link to full text
—
Lampertico P, Bogomolov P, Chulanov V, Stepanova T, Morozov V, Allweiss L, et al. Phase 2 randomised study of bulevirtide as monotherapy or combined with peg-IFNα-2a as treatment for chronic hepatitis delta. Liver Int. 2025 Feb;45(2):e70008. doi: 10.1111/liv.70008 Link to full text
—
Sandmann L, Bremer B, Deterding K, Port K, Gey B, Früchtel C, et al. Letter to the editor: The WHO HDV RNA international standard does not reflect variability of real-world samples. Hepatology. 2025 Feb 1;81(2):E32–E33. doi: 10.1097/HEP.0000000000000975 Link to full text
—
Degasperi E, Sandmann L, Wedemeyer H, Lampertico P; Delta Cure 2024 Working Group. Hepatitis D virus infection: Pathophysiology, epidemiology and treatment. Report from the third Delta Cure Meeting 2024. Liver Int. 2025 Jul;45(7):e70189. doi: 10.1111/liv.70189 Link to full text
—
Sandmann L, Windzio L, Bremer B, Falak S, Beheim-Schwarzbach J, Kummrow A, et al. Droplet digital PCR: A powerful tool for accurate quantification of hepatitis D virus RNA levels and verification of detection limits. J Viral Hepat. 2025 Jun;32(6):e70036. doi: 10.1111/jvh.70036 Link to full text
—
Eichholz JC, Dietz-Fricke C, Mrowietz SK, Dinkelborg K, Port K, Wedemeyer H, et al. Quality of life improves during antiviral therapy with bulevirtide. Z Gastroenterol. 2025 Sep;63(09):930–941. doi:10.1055/a-2633-6361 Link to full text
2024
Jachs M, Sandmann L, Hartl L, Tergast T, Schwarz M, Bauer DJM, et al. Validation of Baveno VII criteria and other non-invasive diagnostic algorithms for clinically significant portal hypertension in hepatitis delta. J Hepatol. 2024 Aug;81(2):248–57. doi:10.1016/j.jhep.2024.03.005 Link to full text
—
Anolli MP, Renteria SU, Degasperi E, Facchetti F, Sambarino D, Borghi M, et al. Comparing methods for plasma HDV RNA quantification in bulevirtide-treated and untreated patients with HDV. JHEP Rep. 2024 Dec 11;7(3):101299. doi:10.1016/j.jhepr.2024.101299 Link to full text
—
Toniutto P, Falleti E, Cmet S, Cussigh A, Degasperi E, Anolli MP, et al. Sodium taurocholate cotransporting polypeptide (NTCP) polymorphisms may influence HDV RNA load and early response to bulevirtide. J Hepatol. 2024 Nov;81(5):819–826. doi:10.1016/j.jhep.2024.06.013 Link to full text
—
Dietz-Fricke C, Degasperi E, Jachs M, Maasoumy B, Reiter FP, Geier A, et al. Safety and efficacy of off-label bulevirtide monotherapy in HDV patients with decompensated Child-B cirrhosis – a real world case series. Hepatology. 2024 Sep 1;80(3):664–673. doi:10.1097/HEP.0000000000000847 Link to full text
—
Sandmann L, Degasperi E, Port K, Aleman S, Wallin JJ, Manuilov D, et al. Liver stiffness measurement as a noninvasive method for the diagnosis of liver cirrhosis in patients with chronic hepatitis D virus infection. Aliment Pharmacol Ther. 2024 Mar;59(6):752–761. doi:10.1111/apt.17878 Link to full text
—
Wranke A, Lobato C, Ceausu E, Dalekos GN, Rizzetto M, Turcanu A, et al. Long-term outcome of hepatitis delta in different regions world-wide: Results of the Hepatitis Delta International Network. Liver Int. 2024 Sep;44(9):2442–2457. doi:10.1111/liv.16006 Link to full text
—
Tonnerre P, Baumert T. Unraveling the liver antiviral immunity in functional cure of chronic hepatitis B using scRNAseq. J Hepatol. 2024 Jul;81(1):14–16. doi:10.1016/j.jhep.2024.03.017 Link to full text
—
Mukherji A, Jühling F, Simanjuntak Y, Crouchet E, Del Zompo F, et al. An atlas of the human liver diurnal transcriptome and its perturbation by hepatitis C virus infection. Nat Commun. 2024 Aug 29;15(1):7486. doi:10.1038/s41467‑024‑51698‑8 Link to full text
—
Zhang B, Li Z, Yang Z, He X, Backes M, et al. SecurityNet: Assessing Machine Learning Vulnerabilities on Public Models. 33rd USENIX Security Symposium, USENIX Association; 2024. pp. 3873–3890. doi:10.48550/arXiv.2310.12665 Link to full text
—
Anastasiou OE, Caruntu FA, Curescu MG, Yalcin K, Akarca US, Gürel S, et al. Five-year follow-up of 96 weeks peginterferon plus tenofovir disoproxil fumarate in hepatitis D. Liver Int. 2024 Jan;44(1):139–147. doi:10.1111/liv.15745 Link to full text
2023
Wedemeyer H, Aleman S, Brunetto MR, Blank A, Andreone P, Bogomolov P, et al. A Phase 3, randomized trial of bulevirtide in chronic hepatitis D. N Engl J Med. 2023 Jul 6;389(1):22–32. doi:10.1056/NEJMoa2213429 Link to full text
—
Sandmann L, Berg T, Deterding K, Fischer N, Hinrichsen H, Petersen J, et al. Antiviral therapy of chronic hepatitis D virus infection – Addendum to the S3 guideline “Prophylaxis, diagnosis and therapy of hepatitis B virus infection” of the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS). AWMF Register Number: 021-11. doi:10.1055/a-2181-3345 Link to full text
—
Dinkelborg K, Kahlhöfer J, Dörge P, Yurdaydin C, Hardtke S, Caruntu FA, et al. Quality-of-life scores improve after 96 weeks of PEG-IFNa-2a treatment of hepatitis D: An analysis of the HIDIT-II trial. Liver Int. 2023 Aug;43(8):1663–1676. doi:10.1111/liv.15602 Link to full text
—
Deterding K, Xu C, Port K, Dietz-Fricke C, Xun J, Maasoumy B, et al. Bile acid increase during bulevirtide treatment of hepatitis D is not associated with a decline in HDV RNA. J Viral Hepat. 2023 Jul;30(7):597–606. doi:10.1111/jvh.13831 Link to full text
—
Wranke A, Heidrich B, Deterding K, Hupa-Breier KL, Kirschner J, Bremer B, et al. Clinical long-term outcome of hepatitis D compared to hepatitis B monoinfection. Hepatol Int. 2023 Dec;17(6):1359–1367. doi:10.1007/s12072-023-10575-0 Link to full text
—
Lampertico P, Degasperi E, Sandmann L, Wedemeyer H, Delta Cure 2022 Working Group. Hepatitis D virus infection: Pathophysiology, epidemiology and treatment. Report from the first international delta cure meeting 2022. JHEP Rep. 2023 Jun 28;5(9):100818. doi:10.1016/j.jhepr.2023.100818 Link to full text
—
Giannone F, Slovic N, Pessaux P, Schuster C, Baumert TF, Lupberger J. Inflammation-related prognostic markers in resected hepatocellular carcinoma. Front Oncol. 2023 Dec 7;13:1267870. doi:10.3389/fonc.2023.1267870 Link to full text
—
Kamal H, Aleman S, D-SOLVE Consortium. Natural history of untreated HDV patients: Always a progressive disease? Liver Int. 2023 Aug;43 Suppl 1:5–21. doi:10.1111/liv.15467 Link to full text
—
Faure‑Dupuy S, Baumert T. Targeting immuno‑metabolism and anti‑viral immune responses in chronic hepatitis B. Hepatol Int. 2023 Oct;17(5):1075–1078. doi:10.1007/s12072‑023‑10546‑5. Link to full text
—
—
Dietz-Fricke C, Tacke F, Zöllner C, Demir M, Schmidt HH, Schramm C, et al. Treating hepatitis D with bulevirtide – Real-world experience from 114 patients. JHEP Rep. 2023 Mar 15;5(4):100686. doi:10.1016/j.jhepr.2023.100686 Link to full text